HPV testing methods
- Rapid test kit
Use urine as a sample, add urine directly to the sample hole of the kit, wait for a few minutes, and observe the results in the kit window. This method is non-invasive and simple to operate. It can be used as a daily test by yourself without the assistance of professionals. If the test kit shows a positive result, conduct a more careful test as soon as possible and treat it as soon as possible.
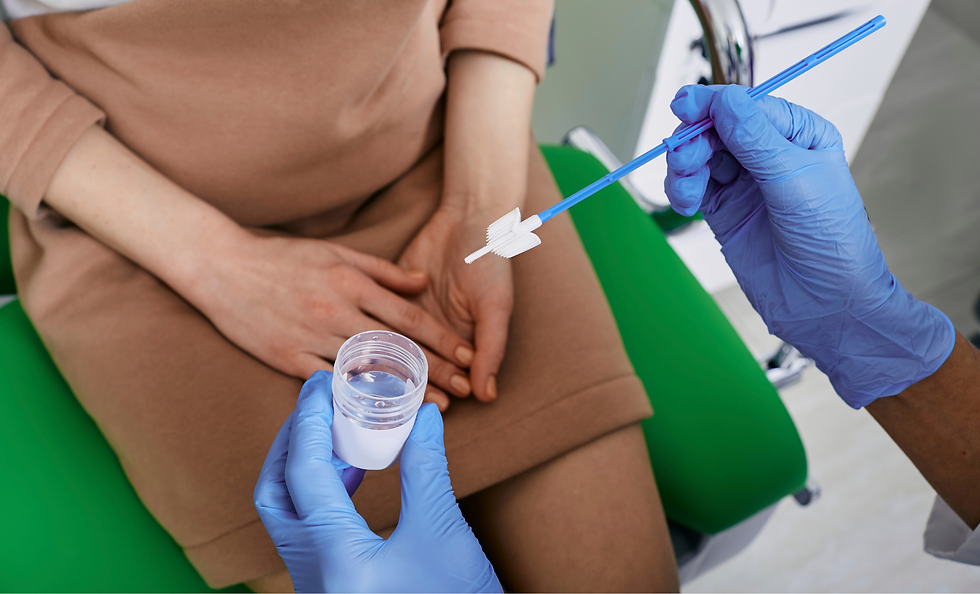
- Cervical smear
The doctor will use a small brush to obtain a cell sample from the cervix, put the cervical cell sample on a smear, and send it to a medical laboratory for microscopic examination to detect cervical cancer or precancerous lesions. If the cervical smear test is abnormal, a more detailed test should be performed as soon as possible, such as another biopsy for confirmation. However, this test cannot tell the type of HPV, and the "false negative" result is as high as 20-30%. This test recommends that women who have had sexual experience should have a cervical smear test once a year. If the test results are normal for two consecutive years, it can be done every three years thereafter.
- HPV virus gene detection
The current HPV virus gene detection is a fluorescent quantitative PCR reaction test. The fluorescent quantitative PCR reaction test is a highly specific primer and probe designed to amplify and detect the target gene, which can monitor the concentration changes of different HPV genotypes in real time. This test can detect almost all high-risk HPV types that cause cancer, as well as most low-risk HPV types that cause cancer. The sensitivity and specificity of this test are relatively high.
Zhang Boliang. [Application Research of Urinary Sulfhydryl Group and HPV Detection in Cervical Cancer Screening.] Maternal and Child Health Care of China, 2013, 25: 4254-4256.
Liu FenFen, L. F., Yu HaiYing, Y. H., Zhao DeYu, Z. D., Hu ShiLian, H. S., Cheng Min, C. M., Xu TingJuan, X. T., & Shen GuoDong, S. G. [Clinical value of urine thiol detection in cervical cancer and precancerous lesion screening.] Chinese Journal of Clinical Healthcare, 2017, Vol. 20, No. 1, 11-13
Mehta V, Vasanth V, Balachandran C. Pap smear. Indian J Dermatol Venereol Leprol 2009;75:214-216
Hubbard, R. A. (2003). Human papillomavirus testing methods. Archives of Pathology & Laboratory Medicine, 127(8), 940–945. https://doi.org/10.5858/2003-127-940-HPTM
Yu, D., Chen, Y., Wu, S., Wang, B., Tang, Y.-W., & Li, L. (2012). Simultaneous Detection and Differentiation of Human Papillomavirus Genotypes 6, 11, 16 and 18 by AllGlo Quadruplex Quantitative PCR. PLoS ONE, 7(11), e48972. https://doi.org/10.1371/journal.pone.0048972